
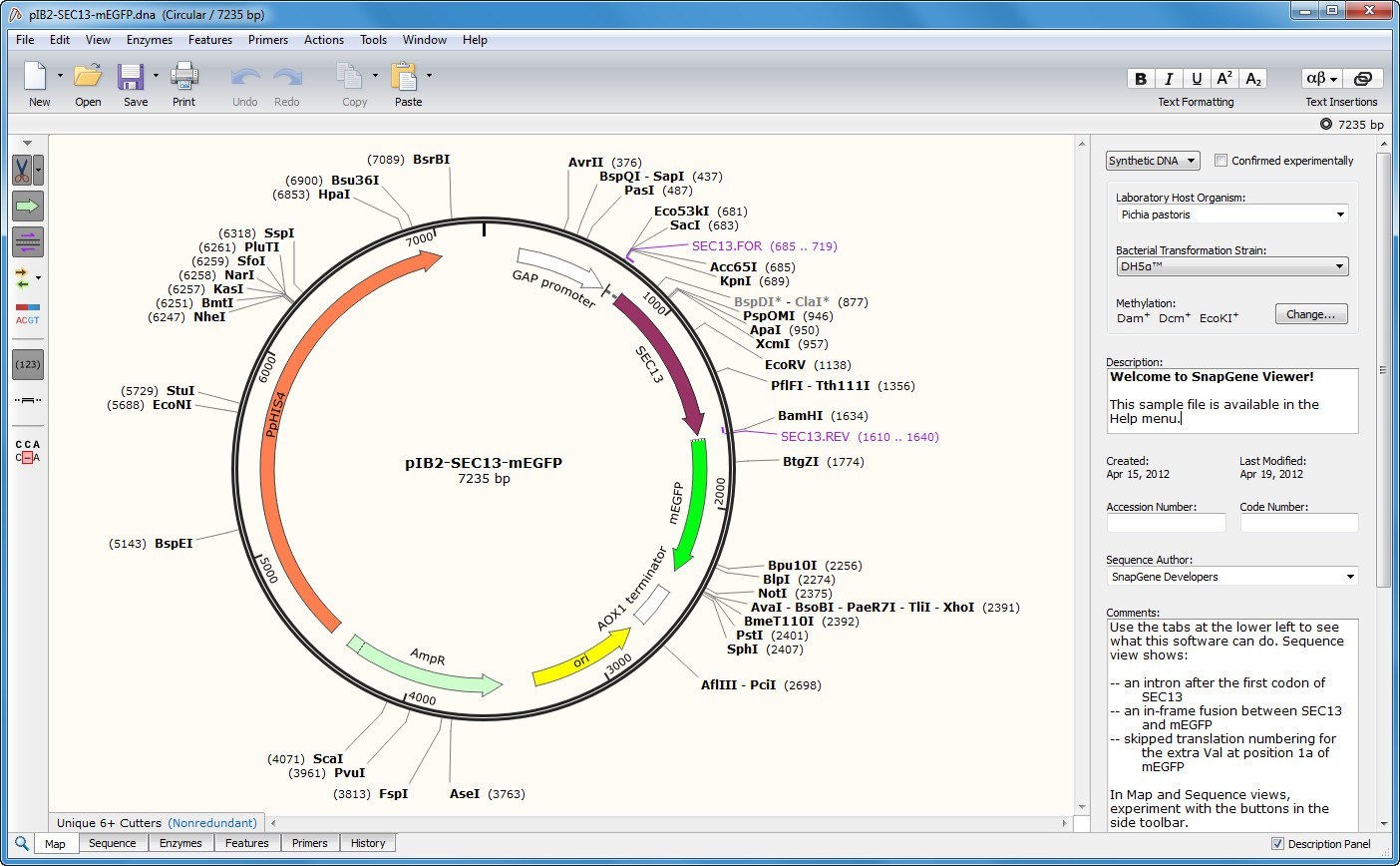
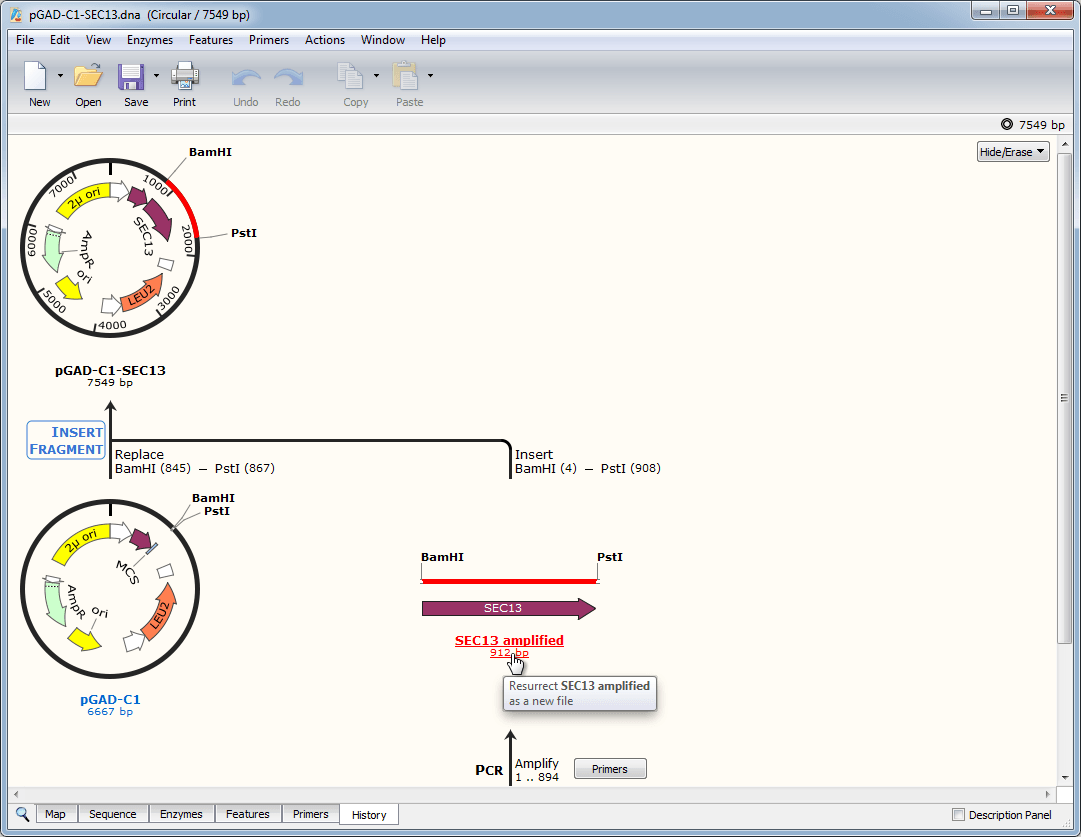
The increasing demand for high amounts of biologics requires continuous optimization and improvement of production technologies. These biotherapeutics, monoclonal antibodies in particular, have shown remarkable market growth in the past few decades. Recombinant proteins used in biomedical research, diagnostics and different therapies are mostly produced in Chinese hamster ovary cells in the pharmaceutical industry. Altogether, our findings point to the feasibility of engineering a fully mapped multi-copy recombinant protein 'production island' in a mammalian cell line with greatly reduced screening effort, improved stability, and predictable product titers. Further, we stably integrate these cassettes into a pre-validated genomic locus. Transient transfections in CHO cells indicates that protein expression increases with the gene copy number on the scaffold. We show a computational workflow for coding and regulatory sequence diversification and optimization followed by experimental assembly of up to nine gene copies and a sentinel reporter on a contiguous scaffold. Key to this strategy is the diversification, at the sequence level, of the individual gene cassettes without altering their protein products. In this study, we present a novel strategy for the rapid design, construction, and genomic integration of engineered multiple-copy gene constructs consisting of up to 10 gene expression cassettes. Alternative approaches for transgene dosage increase and integration are therefore highly desirable. Due to considerable variability in transgene integration profiles, this workflow results in laborious screening campaigns before stable producers can be identified. Current protocols rely on random transgene integration and amplification. The technologies highlighted in this review cover brief outline of the recent animal cell technologies related to industrial and medical applications.Ĭell line development is a critical step in the establishment of a biopharmaceutical manufacturing process. We also review tissue engineering technology because tissue engineering is one of the main exits for mass-produced cells in combination with genetic engineering technology, it can prove to be a promising treatment for patients with genetic diseases after the establishment of induced pluripotent stem cell technology. We further review technologies relating to bioreactors used in the context of animal cells because they are essential for the mass production of target products. We next review genetic engineering technology focusing on CRISPR-Cas system as well as surrounding technologies as these methods have been gaining increasing attention in areas that use animal cells. We review recent advance in mammalian cell line development because this is the first step in the production of recombinant proteins, and it largely affects the efficacy of the production. In this article, we review animal cell technologies and the use of animal cells, focusing on useable cell generation and large-scale production of animal cells.
Furthermore, animal cells are now classified as products because a large number of cells are required for use in regenerative medicine. To produce high-value bioproducts such as antibodies that require glycosylation modification for better performance, animal cells have been recently gaining attention in bioengineering because microorganisms are unsuitable for producing such substances. The industrial use of living organisms for bioproduction of valued substances has been accomplished mostly using microorganisms.


 0 kommentar(er)
0 kommentar(er)
